For Healthcare Professionals
SPIOLTO® RESPIMAT® re-usable
2.5 microgram/2.5 microgram, inhalation solution
tiotropium/olodaterol
- 1. NAME OF THE MEDICINAL PRODUCT
- 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
- 3. PHARMACEUTICAL FORM
- 4. CLINICAL PARTICULARS
- 4.1 THERAPEUTIC INDICATIONS
- 4.2 POSOLOGY AND METHOD OF ADMINISTRATION
- 4.3 CONTRAINDICATIONS
- 4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE
- 4.5 INTERACTION WITH OTHER MEDICINAL PRODUCTS AND OTHER FORMS OF INTERACTION
- 4.6 FERTILITY, PREGNANCY AND LACTATION
- 4.7 EFFECTS ON ABILITY TO DRIVE AND USE MACHINES
- 4.8 UNDESIRABLE EFFECTS
- 4.9 OVERDOSE
- 5. PHARMACOLOGICAL PROPERTIES
- 6. PHARMACEUTICAL PARTICULARS
- 7. MARKETING AUTHORISATION HOLDER/MANUFACTURER
- 8. MARKETING AUTHORISATION NUMBER
- 9. DATE OF REVISION OF THE TEXT
- 10. SPIOLTO® RESPIMAT® re-usable Instructions For Use
- 11. Prepare for first use
- 12. Daily use
- 13. Answers to Common Questions
Instructions for Use Videos
Preparation for first use
Daily use
When to replace your Respimat inhaler
How to replace a cartridge

1. NAME OF THE MEDICINAL PRODUCT
Spiolto® Respimat® re-usable solution for inhalation 2.5 microgram / 2.5 microgram

2. QUALITATIVE AND QUANTITATIVE COMPOSITION
The SPIOLTO RESPIMAT re-usable is a soft mist inhaler delivering tiotropium + olodaterol inhalation solution.
The delivered dose is 2.5 microgram tiotropium and 2.5 microgram olodaterol per puff (2 puffs comprise one medicinal dose) and is equivalent to 3.124 microgram tiotropium bromide monohydrate and 2.7 microgram olodaterol hydrochloride.
(INN = tiotropium bromide)
(INN = olodaterol)
The delivered dose is the dose which is available for the patient after passing the mouthpiece.
For the full list of excipients, see section 6.1

3. PHARMACEUTICAL FORM
Inhalation solution
Clear, colourless, inhalation solution

4. CLINICAL PARTICULARS

4.1 Therapeutic indications
SPIOLTO RESPIMAT re-usable is indicated as a maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD).

4.2 Posology and method of administration
The recommended dose for adults is 5 microgram tiotropium and 5 microgram olodaterol given as two puffs from the Respimat inhaler once daily at the same time of the day (see Handling Instructions).
Elderly
Elderly patients can use SPIOLTO RESPIMAT re-usable at the recommended dose.
Hepatic impairment and renal impairment
SPIOLTO RESPIMAT re-usable contains tiotropium which is a predominantly renally excreted drug and olodaterol, which is predominantly metabolized in the liver.
Hepatic impairment
Patients with mild and moderate hepatic impairment can use SPIOLTO RESPIMAT re-usable at the recommended dose.
There are no data available for use of olodaterol in patients with severe hepatic impairment.
Renal impairment
Renally impaired patients can use SPIOLTO RESPIMAT re-usable at the recommended dose.
SPIOLTO RESPIMAT re-usable contains tiotropium, which is a predominantly renally excreted drug. For patients with moderate to severe impairment (creatinine clearance ≤ 50 ml/min, see Special warnings and precautions for use and Pharmacokinetic properties).
Paediatric population
There is no relevant use of SPIOLTO RESPIMAT re-usable in the paediatric population in COPD. The safety and effectiveness of SPIOLTO RESPIMAT re-usable in the paediatric population have not been established.

4.3 Contraindications
SPIOLTO RESPIMAT re-usable is contraindicated in patients with hypersensitivity to tiotropium or olodaterol or to any of the excipients.
SPIOLTO RESPIMAT re-usable is also contraindicated in patients with a history of hypersensitivity to atropine or its derivatives, e.g. ipratropium or oxitropium.

4.4 Special warnings and precautions for use
General Warnings
SPIOLTO RESPIMAT re-usable should not be used more frequently than once daily.
Asthma
SPIOLTO RESPIMAT re-usable should not be used in asthma. The efficacy and safety of SPIOLTO RESPIMAT re-usable in asthma have not been studied.
The long-term efficacy and safety of olodaterol in the treatment of asthma have not been studied.
LABAs may increase the risk of asthma-related hospitalisations and death.
Acute bronchospasm
SPIOLTO RESPIMAT re-usable is not indicated for the treatment of acute episodes of bronchospasm, i.e. as rescue therapy.
Hypersensitivity
As with all medications, immediate hypersensitivity reactions may occur after administration of SPIOLTO RESPIMAT re-usable.
Paradoxical bronchospasm
As with other inhaled medicines SPIOLTO RESPIMAT re-usable may result in paradoxical bronchospasm that may be life-threatening. If paradoxical bronchospasm occurs SPIOLTO RESPIMAT re-usable should be discontinued immediately and alternative therapy substituted.
Narrow-angle glaucoma, prostatic hyperplasia or bladder-neck obstruction
Consistent with the anticholinergic activity of tiotropium, SPIOLTO RESPIMAT re-usable should be used with caution in patients with narrow-angle glaucoma, prostatic hyperplasia or bladder- neck obstruction.
Patients with renal impairment
As plasma concentration of tiotropium bromide increases with decreased renal function in patients with moderate to severe renal impairment (creatinine clearance ≤ 50 ml/min), SPIOLTO RESPIMAT re-usable should be used only if the expected benefit outweighs the potential risk. There is no long term experience in patients with severe renal impairment (see Pharmacokinetic properties).
Eye symptoms
Patients should be cautioned to avoid getting the spray into their eyes. They should be advised that this may result in precipitation or worsening of narrow-angle glaucoma, eye pain or discomfort, temporary blurring of vision, visual halos or coloured images in association with red eyes from conjunctival congestion and corneal oedema. Should any combination of these eye symptoms develop, patients should stop using SPIOLTO RESPIMAT re-usable and consult a specialist immediately.
Dental caries
Dry mouth, which has been observed with anti-cholinergic treatment, may in the long term be associated with dental caries.
Systemic effects
SPIOLTO RESPIMAT re-usable contains a long acting beta2-adrenergic agonist. Long acting beta2- adrenergic agonists should be administered with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, hypertrophic obstructive cardiomyopathy and hypertension; in patients with convulsive disorders or thyrotoxicosis, in patients with known or suspected prolongation of the QT interval; and in patients who are unusually responsive to sympathomimetic amines.
Cardiovascular effects
Like other beta2-adrenergic agonists, olodaterol may produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, blood pressure, and/or symptoms. In case such effects occur, treatment may need to be discontinued. In addition, beta-adrenergic agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave and ST segment depression, although the clinical significance of these observations is unknown.
Patients with a history of myocardial infarction during the previous year, unstable or life-threatening cardiac arrhythmia, hospitalized for heart failure during the previous year or with a diagnosis of paroxysmal tachycardia (>100 beats per minute) were excluded from the clinical trials. Therefore the experience in these patient groups is limited. SPIOLTO RESPIMAT re-usable should be used with caution in these patient groups.
Hypokalaemia
Beta2-adrenergic agonists may produce significant hypokalaemia in some patients, which has the potential to produce adverse cardiovascular effects. The decrease in serum potassium is usually transient, not requiring supplementation. In patients with severe COPD, hypokalemia may be potentiated by hypoxia and concomitant treatment (please refer to section Interaction with other medicinal products and other forms of interaction), which may increase the susceptibility to cardiac arrhythmias.
Hyperglycaemia
Inhalation of high doses of beta2-adrenergic agonists may produce increases in plasma glucose.
SPIOLTO RESPIMAT re-usable should not be used in conjunction with any other medication containing long-acting beta2-adrenergic agonists or long-acting muscarinic antagonists. Patients who have been taking inhaled, short acting beta2-adrenergic agonists on a regular basis (e.g. four times a day) should be instructed to use them only for symptomatic relief of acute respiratory symptoms.
Benzalkonium Chloride
This medicine contains 0.0011 mg benzalkonium chloride in each actuation.
Benzalkonium chloride may cause wheezing and breathing difficulties. Patients with asthma are at an increased risk for these adverse events.

4.5 Interaction with other medicinal products and other forms of interaction
Although no formal drug interaction studies have been performed, SPIOLTO RESPIMAT re-usable has been used concomitantly with other drugs commonly used in the treatment of COPD, including, short acting sympathomimetic bronchodilators and inhaled corticosteroids without clinical evidence of drug interactions.
Anticholinergic agents
The co-administration of tiotropium bromide, one component of SPIOLTO RESPIMAT re-usable, with other anticholinergic containing drugs has not been studied and therefore is not recommended.
Adrenergic agents
Concomitant administration of other adrenergic agents may potentiate the undesirable effects of SPIOLTO RESPIMAT re-usable.
Xanthine Derivatives, Steroids or Diuretics
Concomitant treatment with xanthine derivatives, steroids, or non-potassium sparing diuretics may potentiate any hypokalaemic effect of adrenergic agonists (see Special warnings and precautions for use).
Beta-blockers
Beta –adrenergic blockers may weaken or antagonize the effect of olodaterol. Cardioselective beta-blockers could be considered, although they should be administered with caution.
MAO Inhibitors, Tricyclic Antidepressants, QTc prolonging drugs
Monoamine oxidase inhibitors, or tricyclic antidepressants or other drugs known to prolong the QTc interval may potentiate the action of SPIOLTO RESPIMAT re-usable on the cardiovascular system.
Pharmacokinetic Drug-Drug interactions
In a drug interaction study with olodaterol using the strong dual CYP and P-gp inhibitor ketoconazole a 1.7-fold increase of systemic exposure was observed (see section Pharmacokinetic properties). No safety concerns were identified in clinical studies of up to one year with olodaterol at doses up to twice the recommended therapeutic dose. No dose adjustment of SPIOLTO RESPIMAT re-usable is necessary.

4.6 Fertility, pregnancy and lactation
Pregnancy
Tiotropium
There is a limited amount of data from the use of tiotropium in pregnant women.
Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity at clinically relevant doses (see section Toxicology).
Olodaterol
For olodaterol no clinical data on exposed pregnancies are available. Preclinical data for olodaterol revealed effects typical for beta-adrenergic agonists at high multiples of the therapeutic doses (please refer to section Toxicology).
The inhibitory effect of beta-adrenergic agonists, like olodaterol a component of SPIOLTO RESPIMAT re-usable on uterine contraction should be taken into account.
As a precautionary measure, it is preferable to avoid the use of SPIOLTO RESPIMAT re-usable during pregnancy.

Lactation
Clinical data from nursing women exposed to tiotropium and/or olodaterol are not available.
In preclinical studies for both tiotropium and olodaterol the substances and/or its metabolites have been detected in the milk of lactating rats, but it is not known whether tiotropium and/or olodaterol pass into human breast milk.
A decision on whether to continue/discontinue breast-feeding or to continue/discontinue therapy with SPIOLTO RESPIMAT re-usable should be made taking into account the benefit of breast-feeding to the child and the benefit of SPIOLTO RESPIMAT re-usable therapy to the woman.

Fertility
Clinical data on fertility are not available for tiotropium and olodaterol or the combination of both components. Preclinical studies performed with the individual components tiotropium and olodaterol showed no indication of any adverse effect on fertility (please refer to section Toxicology).

4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed.
However, patients should be advised that dizziness and blurred vision have been reported with the use of SPIOLTO RESPIMAT re-usable. Therefore, caution should be recommended when driving a car or operating machinery. If patients experience such symptoms they should avoid potentially hazardous tasks such as driving or operating machinery.

4.8 Undesirable effects
The clinical development program of SPIOLTO RESPIMAT re-usable encompassed more than 19000 patients with COPD, of which more than 5900 COPD patients received a dose of 5 microgram tiotropium and 5 microgram olodaterol.
Side effects of SPIOLTO RESPIMAT re-usable were primarily identified from data obtained in 2 active-controlled, parallel-group, long-term treatment (52 weeks) clinical trials in COPD patients comparing SPIOLTO RESPIMAT re-usable with tiotropium and olodaterol. Additionally, a third active-controlled, parallel-group, long-term treatment (52 weeks) clinical trial in COPD patients comparing SPIOLTO RESPIMAT re-usable with tiotropium was conducted (Trial 9). In the two pivotal trials (Trials 1 and 2) the overall incidence of AEs in patients treated with SPIOLTO RESPIMAT re-usable was comparable to patients treated with the mono compound olodaterol at a dose of 5 microgram (74% and 76.6%, respectively). In the pooled analysis of all three long-term clinical trials (Trial 1, 2 and Trial 9) the overall incidence of AEs in patients treated with SPIOLTO RESPIMAT re-usable was comparable to patients treated with the mono component tiotropium at a dose of 5 microgram (74.1% and 74.3 % respectively). All undesirable effects previously reported with one of the individual components are considered undesirable effects with SPIOLTO RESPIMAT re-usable and are included in the adverse reactions listed below. In Trial 9, contributing more than 3900 COPD patients treated with SPIOLTO RESPIMAT re-usable, no new side effects were identified. Furthermore, the safety profile was consistent with that documented in the pivotal trials.
Adverse reactions reported in all clinical trials with SPIOLTO RESPIMAT re-usable are shown below according to system organ class.
These also include all adverse reactions previously reported with one of the individual components
Frequency is defined using the following convention:
Very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000), not known (cannot be estimated from the available data)
| System Organ Class | Adverse reaction | Frequency |
| Infections and infestations | Nasopharyngitis | not known |
| Metabolism and nutrition disorders | Dehydration | not known |
| Nervous system disorders | Dizziness | uncommon |
| Insomnia | rare | |
| Headache | uncommon | |
| Eye disorders | Vision blurred | rare |
| Glaucoma | not known | |
| Intraocular pressure increased | not known | |
| Cardiac disorders | Atrial fibrillation | rare |
| Palpitations | rare | |
| Tachycardia | uncommon | |
| Supraventricular tachycardia | rare | |
| Vascular disorders | Hypertension | rare |
| Respiratory, thoracic and mediastinal disorders | Cough | uncommon |
| Dysphonia | rare | |
| Epistaxis | rare | |
| Laryngitis | rare | |
| Pharyngitis | rare | |
| Bronchospasm | rare | |
| Sinusitis | not known | |
| Gastrointestinal disorders | Dry mouth | uncommon |
| Constipation | rare | |
| Gingivitis | rare | |
| Nausea | rare | |
| Oropharyngeal candidiasis | rare | |
| Intestinal obstruction Ileus paralytic | not known | |
| Dental caries | not known | |
| Dysphagia | not known | |
| Gastro-oesophageal reflux disease | not known | |
| Glossitis | not known | |
| Stomatitis | rare | |
| Skin and subcutaneous tissue disorders, Immune system disorders | Angioedema | rare |
| Urticaria | rare | |
| Hypersensitivity | rare | |
| Pruritus | rare | |
| Anaphylactic reaction | not known | |
| Rash | rare | |
| Dry skin | not known | |
| Skin infection and skin ulcer | not known | |
| Musculoskeletal and connective tissue disorders | Back pain1 | rare |
| Arthralgia | rare | |
| Joint swelling | rare | |
| Renal and urinary disorders | Urinary retention | rare |
| Dysuria | rare | |
| Urinary tract infection | not known |
1 undesirable effects reported with SPIOLTO RESPIMAT re-usable, but not with the individual components
Many of the listed undesirable effects can be assigned to either the anticholinergic properties of tiotropium or to the ß-adrenergic properties of olodaterol, the components of SPIOLTO RESPIMAT re-usable.
In addition the occurrence of other undesirable effects related to the beta-adrenergic agonist class, which are not listed above, should be taken into consideration, such as arrhythmia, myocardial ischemia, angina pectoris, hypotension, tremor, nervousness, muscle spasms, fatigue, malaise, hypokalaemia, hyperglycaemia, and metabolic acidosis.

4.9 Overdose
Symptoms
High doses of tiotropium may lead to anticholinergic signs and symptoms.
No relevant adverse events, beyond dry mouth/throat and dry nasal mucosa in a dose- dependent [10 - 40 μg daily] incidence, were observed following 14-day dosing of up to 40 μg tiotropium inhalation solution in healthy subjects with the exception of pronounced reduction in salivary flow from day 7 onwards. No significant undesirable effects have been observed in six long term studies in COPD patients when a daily dose of 10 μg tiotropium inhalation solution was given over 4 - 48 weeks.
An overdose of olodaterol is likely to lead to exaggerated effects typical of beta2-adrenergic agonists, i.e. myocardial ischemia, hypertension or hypotension, tachycardia, arrhythmias, palpitation, dizziness, nervousness, insomnia, anxiety, headache, tremor, dry mouth, muscle spasms, nausea, fatigue, malaise, hypokalaemia, hyperglycaemia and metabolic acidosis.
Therapy
Treatment with SPIOLTO RESPIMAT re-usable should be discontinued. Supportive and symptomatic treatment is indicated. Serious cases should be hospitalized. Use of cardioselective beta-blockers may be considered, but only subject to extreme caution since the use of beta-adrenergic blocker medication may provoke bronchospasm.

5. PHARMACOLOGICAL PROPERTIES

5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Drugs for obstructive airway diseases, adrenergics in combination with anticholinergics
ATC code: R03AL06

Mode of action
Tiotropium, a long acting muscarinic antagonist and olodaterol a long acting beta2- adrenergic are administered together in the SPIOLTO RESPIMAT re-usable soft mist inhaler. These two active ingredients provide additive bronchodilation due to their different mode of action and different locations of the target receptors in the airways.
Tiotropium:
Tiotropium bromide is a long-acting, muscarinic receptor antagonist (LAMA), in clinical medicine often called an anticholinergic. It has a similar affinity to the subtypes of muscarinic receptors M1 to M5. In the airways, inhibition of M3-receptors at the smooth muscle results in relaxation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In pre- clinical in vitro as well as in vivo studies bronchoprotective effects were dose- dependent and lasted longer than 24 hours. The long duration of the effect is likely to be due to its very slow dissociation from M3-receptors, exhibiting a significantly longer dissociation half- life than that seen with ipratropium. As an N-quaternary anticholinergic tiotropium is topically (broncho-) selective when administered by inhalation, demonstrating an acceptable therapeutic range before giving rise to systemic anti-cholinergic effects.
Dissociation from M2-receptors is faster than from M3, which in functional in vitro studies, elicited (kinetically controlled) receptor subtype selectivity of M3 over M2.
The high potency and slow receptor dissociation found its clinical correlate in significant and long-acting bronchodilation in patients with COPD.
The bronchodilation following inhalation of tiotropium is primarily a local effect (on the airways) not a systemic one.
Olodaterol:
Olodaterol has a high affinity and high selectivity to the human beta2-adrenoceptor. In vitro studies have shown that olodaterol has 241-fold greater agonist activity at beta2- adrenoceptors compared to beta1- adrenoceptors and 2299-fold greater agonist activity compared to beta3-adrenoceptors. The compound exerts its pharmacological effects by binding and activation of beta2-adrenoceptors after topical administration by inhalation.
Activation of these receptors in the airways results in a stimulation of intracellular adenyl cyclase, an enzyme that mediates the synthesis of cyclic-3’,5’ adenosine monophosphate (cAMP). Elevated levels of cAMP induce bronchodilation by relaxation of airway smooth muscle cells.
Olodaterol has the pre-clinical profile of a long-acting selective beta2-adrenoceptor agonist (LABA) with a fast onset of action and duration of action of at least 24 hours.
Beta-adrenoceptors are divided into three subtypes, beta1-adrenoceptors predominantly expressed on cardiac muscle, beta2-adrenoceptors predominantly expressed on airway smooth muscle and beta3- adrenoceptors predominantly expressed on adipose tissue. Beta2-agonists cause bronchodilation. Although the beta2-adrenoceptor is the predominant adrenergic receptor in the airway smooth muscle it is also present on the surface of a variety of other cells, including lung epithelial and endothelial cells and in the heart. The precise function of beta2-receptors in the heart is not known, but their presence raises the possibility that even highly selective beta2-adrenergic agonists may have cardiac effects.

Clinical Trials
Effects on cardiac electrophysiology
Tiotropium:
In a dedicated QT study involving 53 healthy volunteers, tiotropium inhalation powder 18 microgram and 54 microgram (i.e. three times the therapeutic dose) over 12 days did not significantly prolong QT intervals of the ECG.
Olodaterol:
The effect of olodaterol on the QT/QTc interval of the ECG was investigated in 24 healthy male and female volunteers in a double-blind, randomised, placebo- and active (moxifloxacin) controlled study. Olodaterol at single doses of 10, 20, 30 and 50 microgram, demonstrated that compared with placebo, the mean changes from baseline in QT interval over 20 minutes to 2 hours after dosing increased dose- dependently from 1.6 (10 microgram olodaterol) to 6.5 ms (50 microgram olodaterol), with the upper limit of the two-sided 90% confidence intervals being less than 10 ms at all dose levels.
The effect of 5 microgram and 10 microgram olodaterol on heart rate and rhythm was assessed using continuous 24-hour ECG recording (Holter monitoring) in a subset of 772 patients in the 48-week, placebo-controlled Phase 3 Trials. There were no dose- or time-related trends or patterns observed for the magnitudes of mean changes in heart rate or premature beats. Shifts from baseline to the end of treatment in premature beats did not indicate meaningful differences between olodaterol 5 microgram, 10 microgram and placebo.
SPIOLTO RESPIMAT re-usable
In two 52-week randomized, double-blind trials using SPIOLTO RESPIMAT re-usable that enrolled 5162 patients with COPD, ECG assessments were performed post-dose on days 1, 85, 169, and 365. In a pooled analysis the number of subjects with changes from baseline-corrected QT interval of >30 msec using both the Bazett (QTcB) and Fredericia (QTcF), corrections of QT for heart rate ranged from 4.9-6.4% (QTcB) and 1.3-4.7% (QTcF) for the SPIOLTO RESPIMAT group compared to 5.0-6.0% (QTcB) and 1.3-4.4% (QTcF) for olodaterol 5 microgram and 5.3-6.5% (QTcB) and 2.1- 4.6% (QTcF) for tiotropium 5 microgram across the assessments conducted.

Clinical efficacy and safety
The Phase III clinical development program for SPIOLTO RESPIMAT re-usable included three randomised, double-blind trials:
(i) two replicate, 52 week parallel group trials comparing SPIOLTO RESPIMAT re-usable with tiotropium 5 microgram and olodaterol 5 microgram (1029 received SPIOLTO RESPIMAT re-usable) [Trials 1 and 2]
(ii) one 6 week cross-over trial comparing SPIOLTO RESPIMAT re-usable with tiotropium 5 microgram and olodaterol 5 microgram and placebo (139 received SPIOLTO RESPIMAT re-usable) [Trial 3]
In these trials, the comparator products, tiotropium 5 microgram, olodaterol 5 microgram and placebo, were administered via the RESPIMAT re-usable inhaler.
All studies included lung function measurements (forced expiratory volume in one second, FEV1). In the 52 week studies, lung function was measured up to 3 hrs post-dose (12 hrs post- dose in a sub-set of patients) and at 23-24 hrs post-dose; the primary lung function efficacy endpoints were change from pre-treatment baseline (response) in FEV1 AUC0-3h and trough FEV1 after 24 weeks. In the 6 week study, lung function was measured up to 12 hrs post-dose and at 22-24 hrs post-dose; the primary efficacy endpoint was FEV1 AUC0-24h response after 6 weeks. The 52 week trials also included the St. George’s Respiratory Questionnaire (SGRQ) as a primary endpoint as a measure of health-related quality of life and the Mahler Transition Dyspnoea Index (TDI) as a key secondary endpoint as a measure of dyspnoea.
Patients enrolled into the Phase III program were 40 years of age or older with a clinical diagnosis of COPD, had a smoking history of more than 10 pack years and had moderate to very severe pulmonary impairment (post-bronchodilator FEV1 less than 80% predicted normal (GOLD Stage 2-4); post-bronchodilator FEV1 to FVC ratio of less than 70%).

Patient characteristics
The majority of the 5162 patients recruited in the global, 52 week trials [Trials 1 and 2] were male (73%), white (71%) or Asian (25%), with a mean age of 64.0 years. Mean post- bronchodilator FEV1 was 1.37 L (GOLD 2 [50%], GOLD 3 [39%], and GOLD 4 [11%]). Mean β2-agonist responsiveness was 16.6% of baseline (0.171 L). Pulmonary medications allowed as concomitant therapy included inhaled steroids [47%] and xanthine ́s [10%].
The 6 week trial [Trial 3] was conducted in Europe and North America. The majority of the 219 recruited patients were male (59%) and white (99%), with a mean age of 61.1 years. Mean post-bronchodilator FEV1 was 1.55 L (GOLD 2 [64%], GOLD 3 [34%], GOLD 4 [2%]). Mean β2- agonist responsiveness was 15.9% of baseline (0.193 L). Pulmonary medications allowed as concomitant therapy included inhaled steroids [41%] and xanthines [4%].
Lung function
In the 52 week trials, SPIOLTO RESPIMAT re-usable, administered once daily in the morning, provided clear improvement in lung function within 5 minutes after the first dose compared to tiotropium 5 microgram (mean increase in FEV1 of 0.137 L for SPIOLTO RESPIMAT re-usable vs. 0.058 L for tiotropium 5 microgram [p<0.0001] and 0.125 L for olodaterol 5 microgram [p=0.16]). In both studies, significant improvements were observed in FEV1 AUC0-3h response and trough FEV1 response after 24 weeks (lung function primary endpoints) for SPIOLTO RESPIMAT re-usable compared to tiotropium 5 microgram and olodaterol 5 microgram (Table 1).
Table 1: Difference in FEV1 AUC0-3h and trough FEV1 response for SPIOLTO RESPIMAT re-usable compared to tiotropium 5 microgram, olodaterol 5 microgram after 24 weeks (Trials 1 and 2)
| FEV1 AUC0-3h response | Trough FEV1 response | |||||||
| Trial 1 | Trial 2 | Trial 1 | Trial 2 | |||||
| n | Mean | N | Mean | n | Mean | n | Mean | |
| SPIOLTO RESPIMAT versus | 522 | -- | 502 | -- | 521 | -- | 497 | -- |
| Tiotropium 5 microgram | 526 | 0.117 L | 500 | 0.103 L | 520 | 0.071 L | 498 | 0.050 L |
| Olodaterol 5 microgram | 525 | 0.123 L | 507 | 0.132 L | 519 | 0.082 L | 503 | 0.088 L |
pre-treatment baseline FEV1: Trial 1 = 1.16 L; Trial 2 = 1.15 L
p≤0.0001 for all comparisons
The increased bronchodilator effects of SPIOLTO RESPIMAT re-usable compared to tiotropium 5 microgram and olodaterol 5 microgram were maintained throughout the 52 week treatment period. SPIOLTO RESPIMAT re-usable also improved morning and evening PEFR (peak expiratory flow rate) compared to tiotropium 5 microgram and olodaterol 5 microgram as measured by patient's daily recordings.
In the sub-set of patients who completed extended lung function measurements up to 12 hrs post- dose, SPIOLTO RESPIMAT re-usable showed a significantly greater FEV1 response compared to tiotropium 5 microgram and olodaterol 5 microgram over the full 24 hour dosing interval (Figure 1, Table 2).
Figure 1: FEV1 profile for SPIOLTO RESPIMAT re-usable, tiotropium 5 microgram and olodaterol 5 microgram over a continuous 24 hour dosing interval after 24 weeks (12 hr PFT sub-set from Trials 1 and 2; combined dataset)

Table 2: Difference in FEV1 for SPIOLTO RESPIMAT re-usable compared to tiotropium 5 microgram and olodaterol 5 microgram over a continuous 24 hour dosing interval after 24 weeks (12 hr PFT sub-set from Trials 1 and 2; combined dataset)
| n | 12 hr average | 24 hr average | |
| SPIOLTO RESPIMAT versus | 167 | ||
| Tiotropium 5 microgram | 160 | 0.123 | 0.106 |
| Olodaterol 5 microgram | 194 | 0.118 | 0.098 |
pre-treatment baseline FEV1 = 1.17 L
p<0.0001 for all comparisons
In the 6 week trial, SPIOLTO RESPIMAT re-usable showed a significantly greater FEV1 response compared to tiotropium 5 microgram, olodaterol 5 microgram and placebo over the full 24 hour dosing interval (Figure 2, Table 3).
Figure 2: FEV1 profile for SPIOLTO RESPIMAT re-usable, tiotropium 5 microgram, olodaterol 5 microgram and placebo over a continuous 24 hour dosing interval after 6 weeks (Trial 3)

Table 3: Difference in FEV1 (L) for SPIOLTO RESPIMAT re-usable compared to tiotropium 5 microgram, olodaterol 5 microgram and placebo over a continuous 24 hour dosing interval after 6 weeks (Trial 3)
| n | 3 hr average | n | 12 hr average | 24 hr average1 | Trough | |
| SPIOLTO RESPIMAT versus | 138 | 138 | ||||
| Tiotropium 5 microgram | 137 | 0.109 | 135 | 0.119 | 0.110 | 0.079 |
| Olodaterol 5 microgram | 138 | 0.109 | 136 | 0.126 | 0.115 | 0.092 |
| Placebo | 135 | 0.325 | 132 | 0.319 | 0.280 | 0.207 |
pre-treatment baseline FEV1 = 1.30 L
1primary endpoint
p<0.0001 for all comparisons
Dyspnoea
After 24 weeks (Trials 1 and 2), SPIOLTO RESPIMAT re-usable significantly improved mean TDI focal score compared to tiotropium 5 microgram and olodaterol 5 microgram (Table 4). More patients treated with SPIOLTO RESPIMAT re-usable had a clinically meaningful improvement in TDI focal score (MCID, defined as a value of at least 1 unit) compared to tiotropium 5 microgram (54.9% vs. 50.6%, p=0.0546) and olodaterol 5 microgram (54.9% vs. 48.2%, p=0.0026).
Table 4: TDI focal score after 24 weeks of treatment (Trials 1 and 2)
| n | Treatment Mean | Difference to SPIOLTO RESPIMAT | |
| Mean (p-value) | |||
| SPIOLTO RESPIMAT | 992 | 1.98 | |
| Tiotropium 5 microgram | 978 | 1.63 | 0.36 (p=0.008) |
| Olodaterol 5 microgram | 984 | 1.56 | 0.42 (p=0.002) |
Rescue Medication Use
Patients treated with SPIOLTO RESPIMAT re-usable used less daytime and night-time rescue salbutamol compared to patients treated with tiotropium 5 microgram and olodaterol 5 microgram (Trials 1 and 2).
Patient Global Rating
Patients treated with SPIOLTO RESPIMAT re-usable perceived a greater improvement in their respiratory condition compared to tiotropium 5 microgram and olodaterol 5 microgram, as measured by a Patient ́s Global Rating (PGR) scale (Trials 1 and 2).
Exacerbations
Tiotropium 5 microgram has previously demonstrated a statistically significant reduction in risk of a COPD exacerbation compared to placebo. COPD exacerbations was included as an additional endpoint in the 52 week pivotal trials (Trials 1 and 2). In the combined dataset, the proportion of patients experiencing a moderate/severe COPD exacerbation was 27.7% for SPIOLTO RESPIMAT re-usable and 28.8% for tiotropium 5 microgram (p=0.39). These studies were not specifically designed to evaluate the effect of treatments on COPD exacerbations.
In a one-year, randomised, double-blind, active-controlled parallel group clinical trial (Trial 9) SPIOLTO RESPIMAT re-usable was compared with tiotropium 5 microgram on COPD exacerbations. All respiratory medications except anticholinergics, long-acting beta-agonists and combinations thereof were allowed as concomitant treatment, i.e. rapidly acting beta-agonists, inhaled corticosteroids and xanthines. The primary endpoint was the annualised rate of moderate to severe COPD exacerbations (3939 patients received SPIOLTO RESPIMAT re-usable and 3941 patients received tiotropium 5 microgram).
The majority of patients were male (71.4%) and Caucasian (79.3%). The mean age was 66.4 years, mean post-bronchodilator FEV1 was 1.187 L (SD 0.381), and 29.4% of patients had a history of clinically important cardiovascular disease.
Moderate to severe exacerbations of COPD were defined as “a complex of lower respiratory events / symptoms (increase or new onset) related to the underlying COPD, with duration of three days or more, requiring a prescription of antibiotics and/or systemic steroids and/or hospitalisation”.
The annualised rate of moderate to severe COPD exacerbations, was 0.97 for tiotropium 5 mcg and 0.90 for SPIOLTO RESPIMAT re-usable resulting in a rate ratio (RR) of 0.93 (99% CI, 0.85-1.02, p=0.0498). The study did not reach p < 0.01, the pre specified significance level of the study.
In addition the annualised rate of hospitalisations due to a COPD exacerbation was 0.20 for tiotropium 5 mcg and 0.18 for SPIOLTO RESPIMAT re-usable resulting in a RR of 0.89 (95% CI 0.76-1.03, p=0.1265).
Beyond this SPIOLTO RESPIMAT re-usable treatment resulted in a 20% lower annualised rate of moderate to severe exacerbation that required treatment with systemic corticosteroids (RR 0.80, 95% CI 0.68-0.94, p=0.0068) and in a 9% lower annualised rate of moderate to severe exacerbation that required treatment with systemic corticosteroids and antibiotics (RR 0.91, 95% Cl 0.83-1.00, p=0.0447). Treatment with SPIOLTO RESPIMAT re-usable did not result in a reduction in the rate of moderate to severe exacerbations treated with antibiotics only (RR 1.07, 95% CI 0.96-1.20, p=0.2062).
Time to all-cause mortality was included as a secondary endpoint in this trial. There was no significant difference in the risk of all-cause mortality between SPIOLTO RESPIMAT re-usable and tiotropium 5 microgram. During the actual treatment period (i.e. on-treatment plus one day) 36 versus 32 deaths were observed (Hazard Ratio (HR) 1.09, 95% CI, 0.67, 1.75, p=0.7357) while during the planned study period (381 days) 107 versus 121 deaths were observed (HR 0.88, 95% CI, 0.68, 1.15, p=0.3485) for SPIOLTO RESPIMAT re-usable and tiotropium 5 microgram, respectively.
The analysis of the additional exacerbation trial (Trial 9) is displayed in Table 5.
Table 5: Effect of SPIOLTO RESPIMAT re-usable on exacerbations (Trial 9)
| Study (NSpiolto, Ntio 5) | Endpoints | Spiolto Respimat | Tiotropium 5 microgram | Ratio | p-value |
| 1-year Ph IIIb exacerbation study (treated set: 3939, 3941) | Annualised rate of COPD exacerbation Moderate to severe |
0.90 | 0.97 | RR 0.93 (0.85, 1.02) 99% CI | 0.0498 |
| Time to first COPD exacerbation Moderate to severe |
Number of patients with event: 1746 | Number of patients with event: 1777 | HR 0.95 (0.87, 1.03) 99% CI | 0.1188 | |
| Annualised rate of hospitalised exacerbations | 0.18 | 0.20 | RR 0.89 (0.76, 1.03) 95% CI | 0.1265 | |
| Time to first hospitalised COPD exacerbation | Number of patients with event: 450 | Number of patients with event: 469 | HR 0.93 (0.82, 1.06) 95% CI | 0.2773 |
Health-related Quality of Life
After 24 weeks (Trials 1 and 2), SPIOLTO RESPIMAT re-usable significantly improved mean SGRQ total score compared to tiotropium 5 microgram and olodaterol 5 microgram (Table 4); improvements were seen in all SGRQ domains. More patients treated with SPIOLTO RESPIMAT re-usable had a clinically meaningful improvement in SGRQ total score (MCID, defined as a decrease of at least 4 units from baseline) compared to tiotropium 5 microgram (57.5% vs. 48.7%, p=0.0001) and olodaterol 5 microgram (57.5% vs. 44.8%, p<0.0001).
Table 6: SGRQ total and domain scores after 24 weeks of treatment (Trials 1 and 2)
| n | Treatment Mean (change from baseline) | Difference from SPIOLTO RESPIMAT | ||
| Mean (p-value) | ||||
| Total score | Baseline | 43.5 | ||
| SPIOLTO RESPIMAT | 979 | 36.7 (-6.8) | ||
| Tiotropium 5 microgram | 954 | 37.9 (-5.6) | -1.23 (p=0.025) | |
| Olodaterol 5 microgram | 954 | 38.4 (-5.1) | -1.69 (p=0.002) | |
| Symptoms | Baseline | 51.9 | ||
| SPIOLTO RESPIMAT | 982 | 42.6 | ||
| Tiotropium 5 microgram | 957 | 45.5 | -2.94 (p=0.0008) | |
| Olodaterol 5 microgram | 958 | 45.0 | -2.48 (p=0.0046) | |
| Activities | Baseline | 58.0 | ||
| SPIOLTO RESPIMAT | 981 | 51.9 | ||
| Tiotropium 5 microgram | 959 | 53.2 | -1.34 (p=0.052) | |
| Olodaterol 5 microgram | 958 | 54.0 | -2.11 (p=0.002) | |
| Impact | Baseline | 32.6 | ||
| SPIOLTO RESPIMAT | 983 | 26.1 | ||
| Tiotropium 5 microgram | 960 | 26.8 | -0.67 (p=0.283) | |
| Olodaterol 5 microgram | 959 | 27.2 | -1.11 (p=0.075) | |
In two additional 12-week (Trials 7 and 8), placebo-controlled clinical trials, SGRQ total score at 12 weeks was also included as primary endpoint as a measure of health-related quality of life.
In the 12-week trials, SPIOLTO RESPIMAT re-usable demonstrated an improvement compared with placebo at week 12 in mean SGRQ total score (primary endpoint) of -4.9 (95%CI: −6.9, −2.9; p<0.0001) and -4.6 (95%CI: −6.5, −2.6; p<0.0001). In a pooled analysis of the 12-week trials, the proportion of patients with a clinically meaningful decrease in SGRQ total score (defined as a decrease of at least 4 units from baseline) at week 12 was greater for SPIOLTO RESPIMAT re-usable (52%) compared with tiotropium 5 microgram (41%; odds ratio: 1.56 (95%CI: 1.17, 2.07), p = 0.0022) and placebo (32%; odds ratio: 2.35 (95%CI: 1.75, 3.16), p < 0.0001).
In Trial 9, treatment with SPIOLTO RESPIMAT re-usable provided improvements in the COPD Assessment Test score (CAT, a measure of health-related quality of life) versus tiotropium 5 microgram at all study visits (adjusted mean difference versus tiotropium from -0.7 (95% CI (-1.0, -0.5)) at day 90 to -0.4 (95% CI (-0.7,-0.1)) at day 360, all p<0.01). In a responder analysis the proportion of patients experiencing a clinically meaningful improvement in CAT (defined as a reduction of 2 points or more) was larger with SPIOLTO RESPIMAT re-usable versus tiotropium 5 microgram (44.51% vs 40.77% respectively, odds ratio 1.17, 95% CI 1.06-1.28 p<0.001).
Inspiratory capacity, breathing discomfort and exercise endurance
The effect of SPIOLTO RESPIMAT re-usable on inspiratory capacity, breathing discomfort and symptom-limited exercise endurance was investigated in three randomised, double-blind trials in COPD patients:
(i) two replicate, 6 week cross-over trials comparing SPIOLTO RESPIMAT re-usable with tiotropium 5 microgram, olodaterol 5 microgram and placebo during constant work rate cycling (450 received SPIOLTO RESPIMAT re-usable) [Trials 4 and 5]
(ii) one 12 week parallel group trial comparing SPIOLTO RESPIMAT re-usable with placebo during constant work rate cycling (139 received SPIOLTO RESPIMAT re-usable) and constant speed walking (sub-set of patients) [Trial 6]
SPIOLTO RESPIMAT re-usable significantly improved inspiratory capacity at rest two hours post-dose compared to tiotropium 5 microgram (0.114 L, p<0.0001; Trial 4, 0.088 L, p=0.0005; Trial 5), olodaterol 5 microgram (0.119 L, p<0.0001; Trial 4, 0.080 L, p=0.0015; Trial 5) and placebo (0.244 L, p<0.0001; Trial 4, 0.265 L, p<0.0001; Trial 5) after 6 weeks.
In Trials 4 and 5, SPIOLTO RESPIMAT re-usable significantly improved endurance time during constant work rate cycling compared to placebo after 6 weeks (Trial 4: geometric mean endurance time of 454 s for SPIOLTO RESPIMAT re-usable compared to 375 seconds for placebo (20.9% improvement, p<0.0001); Trial 5: geometric mean endurance time of 466 seconds for SPIOLTO RESPIMAT re-usable compared to 411 seconds for placebo (13.4% improvement, p<0.0001).
In Trial 6, SPIOLTO RESPIMAT re-usable significantly improved endurance time during constant work rate cycling compared to placebo after 12 weeks (geometric endurance time of 528 seconds for SPIOLTO RESPIMAT re-usable compared to 464 seconds for placebo (13.8% improvement, p=0.021).

Long-term tiotropium active-controlled study
In the post marketing TIOSPIR study comparing Spiriva Respimat and Spiriva HandiHaler, all cause mortality (including vital status follow up) was similar with hazard ratio (Spiriva Respimat/Spiriva HandiHaler) = (0.96, 95% CI 0.84-1.09).
Respective treatment exposure was 13,135 and 13,050 patient years.

5.2 Pharmacokinetic properties
When tiotropium and olodaterol were administered in combination by the inhaled route, the pharmacokinetic parameters for each component were similar to those observed when each active substance was administered separately.
Tiotropium and olodaterol demonstrate linear pharmacokinetics in the therapeutic range. On repeated once-daily inhalation administration, steady state of tiotropium is reached by day 7. Steady state of olodaterol is achieved after 8 days of once-daily inhalation, and accumulation is up to 1.8-fold as compared to a single dose.

Absorption
Tiotropium: Urinary excretion data from young healthy volunteers suggests that approximately 33% of the dose inhaled via the RESPIMAT re-usable inhaler reaches the systemic circulation. The absolute bioavailability from an orally administered solution was found to be 2–3%. Maximum tiotropium plasma concentrations are observed 5–7 minutes after the inhalation via RESPIMAT.
Olodaterol: In healthy volunteers the absolute bioavailability of olodaterol following inhalation was estimated to be approximately 30%, whereas the absolute bioavailability was below 1% when given as an oral solution. Maximum olodaterol plasma concentrations generally are reached within 10 to 20 minutes following drug inhalation via RESPIMAT.

Distribution
Tiotropium has a plasma protein binding of 72% and shows a volume of distribution of 32 L/kg. Studies in rats have shown that tiotropium does not penetrate the blood-brain barrier to any relevant extent.
Olodaterol has a plasma protein binding of approximately 60% and shows a volume of distribution of 1110 L.

Biotransformation
Tiotropium: The extent of metabolism is small. This is evident from 74% of an intravenous dose being excreted in the urine as unchanged drug. The ester tiotropium is nonenzymatically cleaved into its alcohol and acid component (N-methylscopine and dithienylglycolic acid, respectively), both not binding to muscarinic receptors. In vitro experiments with human liver microsomes and human hepatocytes suggest that some further drug (<20% of the dose after intravenous administration) is metabolized by cytochrome P450 (CYP) 2D6 and 3A4 dependent oxidation and subsequent glutathione conjugation to a variety of Phase II-metabolites.
Olodaterol is substantially metabolized by direct glucuronidation and by O-demethylation at the methoxy moiety followed by conjugation. Of the six metabolites identified, only the unconjugated demethylation product (SOM 1522) binds to ß2-receptors; this metabolite however is not detectable in plasma after chronic inhalation of the recommended therapeutic dose or doses of up to 4-fold higher. Cytochrome P450 isozymes CYP2C9 and CYP2C8, with negligible contribution of CYP3A4, are involved in the O-demethylation of olodaterol, while uridine diphosphate glycosyl transferase isoforms UGT2B7, UGT1A1, 1A7 and 1A9 were shown to be involved in the formation of olodaterol glucuronides.

Elimination
Tiotropium: Intravenously administered tiotropium is mainly excreted unchanged in urine (74%). The total clearance in healthy volunteers is 880 mL/min. After inhalation by COPD patients to steady-state, urinary excretion is 18.6% of the dose, the remainder being mainly non-absorbed drug in gut that is eliminated via the faeces. The renal clearance of tiotropium exceeds the glomerular filtration rate, indicating active secretion into the urine. The effective half-life of tiotropium following inhalation by COPD patients ranges between 27 and 45 h.
Olodaterol: Total clearance of olodaterol in healthy volunteers is 872 mL/min, and renal clearance is 173 mL/min. The terminal half-life following intravenous administration is 22 hrs. The terminal half-life following inhalation in contrast is about 45 hrs, indicating that the latter is determined by absorption rather than by elimination processes.
Following intravenous administration of [14C]-labelled olodaterol, 38% of the radioactive dose was recovered in the urine and 53% was recovered in feces. The amount of unchanged olodaterol recovered in the urine after intravenous administration was 19%. Following oral administration, only 9% of the radioactivity was recovered in urine, while the major portion was recovered in feces (84%). More than 90% of the dose was excreted within 6 and 5 days following intravenous and oral administration, respectively. Following inhalation, excretion of unchanged olodaterol in urine within the dosing interval in healthy volunteers at steady state accounted for 5-7% of the dose.

Characteristics in Patients
Tiotropium: As expected for all predominantly renally excreted drugs, advancing age was associated with a decrease of tiotropium renal clearance from 347 mL/min in COPD patients <65 years to 275 mL/min in COPD patients ≥65 years. This did not result in a corresponding increase in AUC0-6,ss or Cmax,ss values.
Olodaterol: A pharmacokinetic meta-analysis utilizing data from 2 controlled clinical trials that included 405 patients with COPD and 296 patients with asthma showed that no dose adjustment is necessary due to effects of age, gender and weight on systemic exposure to olodaterol.
Comparison of pharmacokinetic data within and across studies with olodaterol revealed a trend for higher systemic exposure in Japanese and other Asians than in Caucasians.
No safety concerns were identified in clinical studies with olodaterol in Caucasians and Asians of up to one year with olodaterol doses up to twice the recommended therapeutic dose.

Renal Insufficiency
Tiotropium: Following once daily inhaled administration of tiotropium to steady-state in COPD patients with mild renal impairment (CLCR 50-80 mL/min) resulted in slightly higher AUC0- 6,ss(between 1.8 to 30% higher) and similar Cmax,ss compared to patients with normal renal function (CLcr >80 mL/min). In subjects with moderate to severe renal impairment (CLCR <50 ml/min) intravenous administration of tiotropium resulted in twofold higher total exposure (82% higher AUC0-4h and 52% higher Cmax) compared to subjects with normal renal function, which was confirmed by observations after dry powder inhalation.
Olodaterol: In subjects with severe renal impairment (CLCR <30 mL/min) systemic exposure to olodaterol was on average 1.4-fold increased. This magnitude of exposure increase does not raise any safety concerns given the safety experience of treatment with olodaterol in clinical studies of up to one year at doses up to twice the recommended therapeutic dose.

Hepatic Insufficiency
Tiotropium: Liver insufficiency is not expected to have any relevant influence on tiotropium pharmacokinetics. Tiotropium is predominantly cleared by renal elimination (74% in young healthy volunteers) and simple non-enzymatic ester cleavage to pharmacologically inactive products.
Olodaterol: In subjects with mild and moderate hepatic impairment systemic exposure to olodaterol was not affected. The effect of severe hepatic impairment on systemic exposure to olodaterol was not investigated.

Drug-Drug Interactions
Pharmacokinetic drug interaction studies with SPIOLTO RESPIMAT re-usable have not been performed; however such studies have been conducted with individual components tiotropium and olodaterol.
When tiotropium and olodaterol were administered in combination by the inhaled route, the pharmacokinetic parameters for each component were similar to those observed when each active substance was administered separately.
Tiotropium
An interaction study with tiotropium (14.4 mcg intravenous infusion over 15 minutes) and cimetidine 400 mg three times daily or ranitidine 300 mg once-daily was conducted. Concomitant administration of cimetidine with tiotropium resulted in a 20% increase in the AUC0-4h, a 28% decrease in the renal clearance of tiotropium and no significant change in the Cmax and amount excreted in urine over 96 hours. Co-administration of tiotropium with ranitidine did not affect the pharmacokinetics of tiotropium.
Common concomitant medications (long-acting beta2-adrenergic agonists (LABA), inhaled corticosterioids (ICS)) used by patients with COPD were not found to alter the exposure to tiotropium.
Olodaterol:
Drug-drug interaction studies were carried out using fluconazole as model inhibitor of CYP 2C9 and ketoconazole as potent P-gp and CYP inhibitor.
Fluconazole:
Co-administration of 400 mg fluconazole once daily for 14 days had no relevant effect on systemic exposure to olodaterol.
Ketoconazole:
Co-administration of 400 mg ketoconazole once daily for 14 days increased olodaterol Cmax by 66% and AUC0-1 by 68%.
Tiotropium:
Co-administration of tiotropium bromide, delivered as a fixed-dose combination with olodaterol, for 21 days had no relevant effect on systemic exposure to olodaterol, and vice versa.

5.3 Preclinical safety data
Tiotropium+Olodaterol
Single-dose toxicity
For the combination tiotropium + olodaterol single-dose toxicity studies after inhalation administration have been performed for three dose ratios in mice and rats, revealing a low acute toxicity. In mice, the approximate lethal doses (ALD) were 34.8+36.6 mg/kg for tiotropium+olodaterol in the ratio 1:1. In rats, no deaths occurred, therefore the ALDs were >17.9+18.8 mg/kg for tiotropium/olodaterol in the ratio 1:1.

Repeat-dose toxicity
Inhalation repeat-dose toxicity studies for the combination tiotropium+olodaterol were performed in rats (4 weeks) and dogs (up to 13 weeks) at different dose ratios. In the 13-week studies in dogs, body weight development, clinical signs, changes of the cardiovascular system and of respective enzyme activities as well as the macroscopical and microscopical pathology were characteristic β2-agonistic and anticholinergic effects. In the 13-week toxicity studies with the dose ratio 1:1 for tiotropium/olodaterol, the no observed adverse effect levels (NOAEL) were 14+16 microgram/kg/day.

Reproduction toxicity
No reproduction toxicity studies for the combination were performed.
Tiotropium:
In the reproduction studies in rabbits and rats harmful effects with respect to pregnancy, embryo/fetal development, parturition or postnatal development could only be demonstrated at maternally toxic dose levels. In a general reproduction and fertility study in rats, there was no indication of any adverse effect on fertility or mating performance of either treated parents or their offspring at any dosage.
Olodaterol:
In rats, no teratogenic effects occurred after inhalation at doses 1054 microgram/kg/day (> 2600 times the human exposure (AUC(0-24h)) at the dose of 5 microgram). In pregnant NZW rabbits, an inhalation dose of 2489 microgram/kg/day (approximately 7130 times the human exposure at 5 microgram based on AUC(0-24h)) of olodaterol exhibited fetal toxicity characteristically resulting from ß-adrenoceptor stimulation; these included patchy ossifications, short/bent bones, partially open eye, cleft palate, cardiovascular abnormalities. No significant effects occurred at a dose of 974 microgram/kg (approximately 1353 times the 5 microgram dose based on AUC(0-24h)). No impairment of male or female fertility or early embryonic development was seen in the rat up to inhalation doses of 3068 microgram/kg (approximately 2332 times the 5 microgram dose based on AUC(0-24h)).
No effects were observed on mating, fertility or bearing of live implants to Day 14/15/16 of gestation in the F1 animals in the rat up to inhalation doses of 3665 microgram/kg/day (approximately 2332 times the 5 microgram dose based on AUC(0-24h)).

Genotoxicity
In vitro mutagenicity for tiotropium or olodaterol alone, did not show any genotoxic potential. In the in vivo rat bone marrow micronucleus assay, after inhalation at dose levels of up to 2266+2174 microgram/kg/day tiotropium+olodaterol for 4 weeks (dose ratio 1:1), the combination was free of genotoxic potential.
In the in vivo rat bone marrow micronucleus assay after inhalation exposure (up to approximately 1092 times the 5 microgram dose based on AUC(0-24h)) and the in vitro (Ames test, mouse lymphoma assay) mutagenicity assays, olodaterol was free of any genotoxic potential up to very high dose levels. An increased frequency of micronuclei was observed in rats after i.v. exposure at doses of at least 5500-times the 5 microgram dose based on AUC(0-24h) may be related to drug enhanced (compensatory) erythropoiesis.

Carcinogenicity
No carcinogenicity studies for the combination were performed.
Tiotropium:
Tiotropium did not show any carcinogenic potential in the respective studies in mice and rats.
Olodaterol:
Lifetime treatment of rats induced class- and rodent-specific leiomyomas of the mesovarium at exposures approximately 2235-fold and 715-fold the exposure at the dose of 5 microgram dose (on systemic exposure). Lifetime treatment of mice induced class- and rodent-specific smooth muscle tumours (leiomyomas, leiomyosarcomas) of the uterus and incidences of sex cord stromal focal hyperplasia and luteal focal hyperplasia in the ovary at exposures approximately 477- to 3596-fold the exposure at the dose of 5 microgram dose (on systemic exposure), again considered as class- and rodent specific (exposure multiples). Both studies revealed no evidence for an olodaterol-related human risk with regard to carcinogenicity or chronic toxicity.

6. PHARMACEUTICAL PARTICULARS

6.1 List of excipients
Benzalkonium chloride
Disodium edetate
Water, purified
1 M hydrochloric acid (for pH adjustment)

6.2 Incompatibilities
Not applicable.

6.3 Shelf life
Please refer to packaging for information on shelf life.

6.4 Special precautions for storage
Store at or below 30°C. Do not freeze

6.5 Nature and contents of container
Type and material of the container in contact with the medicinal product:
Solution filled into a polyethylene/polypropylene cartridge with a polypropylene cap with integrated silicone sealing ring. The cartridge is enclosed within an aluminium cylinder.
Each cartridge contains 4 ml inhalation solution.
Pack sizes and devices supplied:
Single pack: 1 Respimat re-usable inhaler and 1 cartridge, providing 60 puffs (30 medicinal doses)
Single refill pack: 1 cartridge, providing 60 puffs (30 medicinal doses)
Recommended use: 6 cartridges per inhaler
Note: The functioning of the Respimat re-usable inhaler has been demonstrated in tests for 540 actuations (corresponding to 9 cartridges).

6.6 Special precautions for disposal and other handling
Discard cartridge 3 months after insertion

7. MARKETING AUTHORISATION HOLDER/MANUFACTURER
Manufactured by
Boehringer Ingelheim Pharma GmbH & Co. KG
Ingelheim am Rhein
Germany
For
Boehringer Ingelheim International GmbH
Ingelheim am Rhein
Germany

8. MARKETING AUTHORISATION NUMBER
SIN15890P

9. DATE OF REVISION OF THE TEXT
30 May 2024

SPIOLTO RESPIMAT re-usable
HANDLING INSTRUCTIONS
SPIOLTO RESPIMAT re-usable (tiotropium bromide and olodaterol).
Read these Handling Instructions before you start using SPIOLTO RESPIMAT re-usable.
You will need to use this inhaler only ONCE A DAY. Each time you use it take TWO PUFFS.
- If not been used for more than 7 days release one puff towards the ground.
- If not been used for more than 21 days repeat steps 4 to 6 until a cloud is visible. Then repeat steps 4 to 6 three more times.
How to care for your SPIOLTO RESPIMAT re-usable
Clean the mouthpiece including the metal part inside the mouthpiece with a damp cloth or tissue only, at least once a week.
Any minor discoloration in the mouthpiece does not affect your SPIOLTO RESPIMAT re-usable inhaler performance. If necessary, wipe the outside of your SPIOLTO RESPIMAT re-usable inhaler with a damp cloth.
When to replace the inhaler
When you have used an inhaler with 6 cartridges, get a new SPIOLTO RESPIMAT re-usable pack containing an inhaler. Do not use the Respimat re-usable inhaler for more than one year, after having inserted the first cartridge.



Prepare for first use
1. Remove clear base
- Keep the cap closed.
- Press the safety catch while pulling off the clear base with your other hand.

2. Insert cartridge
- Insert the cartridge into the inhaler.
- Place the inhaler on a firm surface and push down firmly until it clicks into place.

3. Track cartridge and put clear base back
- Mark the check-box on inhaler’s label to track the number of cartridges.
- Put the clear base back into place until it clicks.

4. Turn
- Keep the cap closed.
- Turn the clear base in the direction of the arrows on the label until it clicks (half a turn).

5. Open
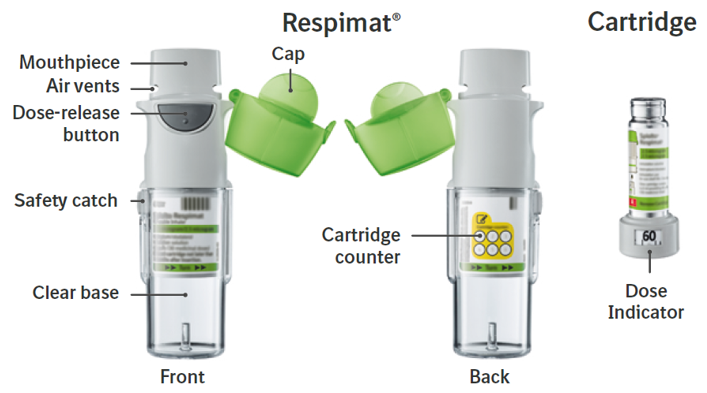
- Open the cap until it snaps fully open.
6. Press
- Point the inhaler toward the ground.
- Press the dose-release button.
- Close the cap.
- Repeat steps 4-6 until a cloud is visible.
- After a cloud is visible, repeat steps 4-6 three more times.
Your inhaler is now ready to use and will deliver 60 puffs (30 doses).


Daily use
TURN
- Keep the cap closed.
- TURN the clear base in the direction of the arrows on the label until it clicks (half a turn).

OPEN
- OPEN the cap until it snaps fully open.

PRESS
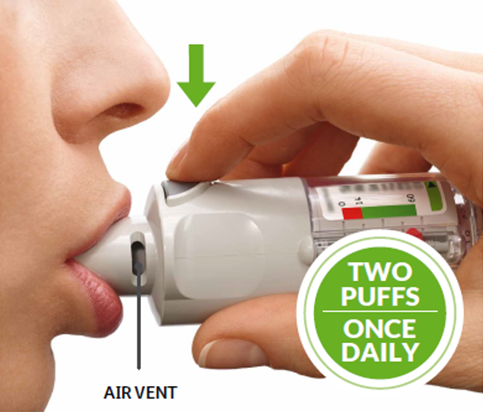
- Breathe out slowly and fully.
- Close your lips around the mouthpiece without covering the air vents. Point your Inhaler to the back of your throat.
- While taking a slow, deep breath through your mouth, PRESS the dose-release button and continue to breathe in slowly for as long as comfortable.
- Hold your breath for 10 seconds or for as long as comfortable.
- Repeat TURN, OPEN, PRESS for a total of 2 puffs.
- Close the cap until you use your inhaler again.
When to replace your Respimat inhaler
How to replace a cartridge
When to replace the SPIOLTO RESPIMAT re-usable cartridge
The dose indication shows how many puffs remain in the cartridge.

60 Puffs remaining

Less than 10 puffs remaining. Obtain a new cartridge

Your cartridge is used up. Turn the clear base to loosen it. Your inhaler is now in a locked position. Pull off the cartridge from the inhaler. Insert a new cartridge until it clicks (refer to step 2). The new cartridge will stick out more than the very first cartridge (continue with step 3). Remember to put the clear base back to unlock the inhaler.

Answers to Common Questions
Did you accidentally turn the clear base before inserting the cartridge?
Open the cap, press the dose-release button, then insert the cartridge.
Are you replacing the cartridge?
The new cartridge will stick out more than the very first cartridge. Insert it until it clicks, then replace the clear base.
Did you put the clear base back?
If not, put the clear base back to unlock the inhaler. The Respimat re-usable only functions with the clear base in place.
Did you turn the clear base?
If not, turn the clear base in a continuous movement until it clicks (half a turn).
Does the dose indicator on your cartridge display a white arrow on a red background?
Your cartridge is used up. Insert a new cartridge.
Pull and turn the cartridge at the same time.
Is the clear base loose and does the dose indicator on your cartridge display a white arrow on a red background?
Your cartridge is used up. Insert a new cartridge.
Did you turn the clear base already?
If the clear base has already been turned, follow steps “OPEN” and “PRESS” under “Daily Use” to get your medicine.
Did you use RESPIMAT re-usable as indicated (two puffs/once daily)?
RESPIMAT re-usable will last 30 days if used at two puffs once daily.
Did you spray in the air often to check whether the RESPIMAT re-usable is working?
Once you have prepared RESPIMAT re-usable, no test-spraying is required if used daily.
Did you take off and put the clear base multiple times back?
Do not remove the clear base before the cartridge is used up. Each time you take off the clear base without cartridge exchange, the dose counter records one puff and the remaining doses are reduced.
Did you insert a cartridge?
If not, insert a cartridge. Once your RESPIMAT re-usable is assembled, do not remove the clear base or the cartridge until the cartridge is used up.
Did you repeat TURN, OPEN, PRESS less than three times after inserting the cartridge?
Repeat TURN, OPEN, PRESS three times after inserting the cartridge as shown in the steps 4 to 6 under “Prepare for use”.
Does the dose indicator on your cartridge display a white arrow on a red background?
Your cartridge is used up. Insert a new cartridge.
Was the cap open when you turned the clear base?
Close the cap, then turn the clear base.
Did you press the dose-release button when turning the clear base?
Close the cap, so the dose-release button is covered, then turn the clear base.
Did you stop when turning the clear base before it clicked?
Turn the clear base in a continuous movement until it clicks (half a turn). The dose counter will count each incomplete turn and the number of remaining doses is reduced.
Was the cap open when you replaced the cartridge?
Close the cap, then replace the cartridge.

